At some point, most people need a dressing – at the very least after blood has been taken or following an injection – and this may remain on the skin for several hours in some cases. Due to this direct skin contact, the quality requirements for these dressings are exceptionally high. To ensure maximum patient safety, the various production steps are monitored by 100% inspections that are synchronised with the machine cycle. This means that contaminated or defective products can be separated out automatically.
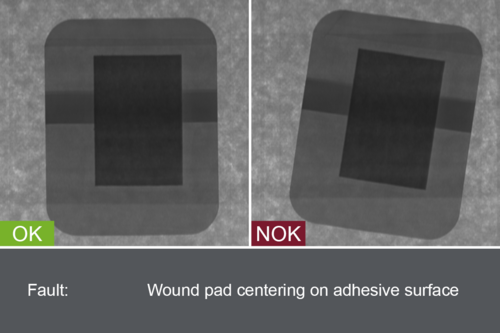
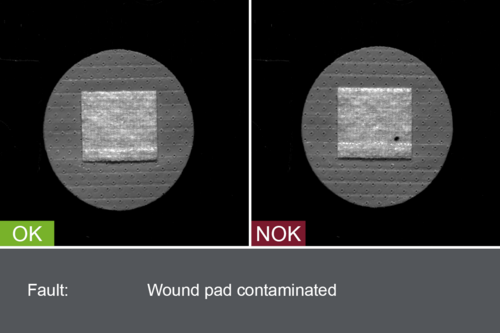
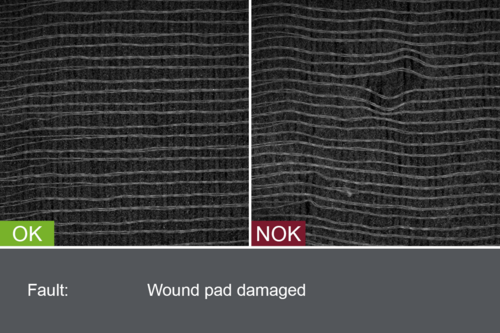
Inspection systems from OCTUM’s patch.inspect series are specially tailor-made for the dressing materials under production and the in-process requirements. Adhesive plasters and Transdermal Therapeutic Plasters (TTS) are examined for material defects, cosmetic flaws and contamination by inline inspection in various stages of production. In addition, the uniform application of active ingredients is also monitored, especially where several coats are involved.
To guarantee maximum product quality before packaging, the release liner, print and seams of the sealed bag are monitored in a final inspection. This way, defective products can be detected at an early stage of production and reliably separated out. patch.inspect systems are compliant with EU GMP and 21 CFR Part 11 and reduce pseudo rejects in the sterile production environment.

Any questions?
Get directly in touch with our
experts on +49 7062 91494-0
or write to us using our contact form
With patch.inspect, many different dressing materials can be automatically inspected:
- Wound dressings: e.g. compresses, packing
- Adhesive bandages: e.g. plasters, sticking plasters
- Medicated plasters: e.g. pain-relieving and anti-inflammatory plasters
- Transdermal patches: e.g. pain relief, hormone replacement therapy, contraception, nicotine patches
- Other dressing materials and packaging possible on request
- Inspection zone:
- Surface of dressing materials (bandages and TTS)
- As strips or pre-cut
- Inspection characteristics:
- Contamination (impurities, foreign bodies)
- Cosmetic defects
- Material defects
- Product condition, shape and size
- Quality of cut
- Application of active ingredients (one or more coats)
- Sealing defects
- Print and code
- Defect sizes: Foreign bodies from 0.2 mm
- Machine cycle time: up to 60 m/min
- EU GAMP and 21 CFR Part 11 compliant
- In a stainless steel case
- Special versions available with UV or IR lighting

![[Translate to Englisch:] medizinisches Personal appliziert ein Pflaster auf den Oberarm des Patienten](/fileadmin/user_upload/content-bilder/Branchenl%C3%B6sungen/Medizintechnik/patch.inspect/Pflaster_800x500_-_Kopfbild.jpg)