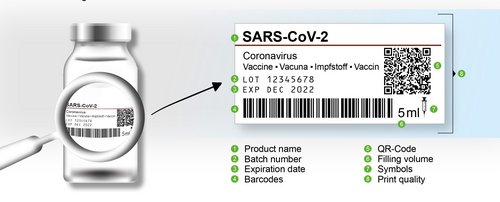
The labelling of medical devices is particularly important: Only the clear labelling and reliable identification of vials, syringes, folding boxes, shipping cartons and pallets enable manufacturers to guarantee seamless traceability of each batch in the event of a recall. Moreover, the labels on medicinal containers – which are generally applied immediately after sealing – contain differing information such as batch and serial numbers and must be absolutely defect-free in order to conform to the strict standards of the pharmaceuticals and medical technology sector.
Inspection systems from OCTUM’s label.inspect series have been designed for reliable label inspection and cover size and colour variations of all kinds. They perform 100% inspection of each individual label and verify the print to ensure that identification is present, legible and contains the correct information. In the interests of patient safety, this provides the basis for unambiguous life cycle documentation in accordance with the necessary medicine standards and fast, seamless and objective traceability of individual batches.

Any questions?
Get directly in touch with our
experts on +49 7062 91494-0
or write to us using our contact form
- Inspects up to 1,000 labels/min
- Suitable for labels of all sizes and colours
- 21 CFR Part 11 and EU GMP compliant
- New sizes can be set up quickly and flexibly
- User management and audit trails
- Modular for easy expansion
- UV ink can also be checked as an option
- Optionally in a stainless steel case with integrated light
Inspection characteristics
Label inspection demands different checks, depending on the task at hand. OCTUM can cover all these, in consultation with the user and in compliance with the required ISO and AIM regulations and GS1 code analysis standards. Below are some tasks as examples:
- Printed text verification
- Verification of all common 1D and 2D codes (barcodes, data matrix, etc.)
- Data verification of batch numbers, serial numbers, date of manufacture, expiry date, etc.
- Check of correct label position
- Print quality inspection
- Comparison with specified values
Area of application
Printed labels are used in a great many sizes and colours in medical technology. What’s more, the content and codes used sometimes differ considerably from one manufacturer to another. label.inspect enables the reliable inspection of labels that are used for products including:
- Vials
- Syringes
- Cardboard boxes
- Bundles
- Films
- and a great deal more
Printing process
Manufacturers of medical devices employ different printing processes for identifying their products. label.inspect covers all popular processes and enables the inspection of labels produced using the following printing methods:
- Laser printing
- Thermal transfer printing
- Inkjet printing

![[Translate to Englisch:] auf einem Karton bzw. einer Verpackung prüft ein Mensch mit seinem Smartphone Codes und Klarschrift auf einem Etikett](/fileadmin/user_upload/content-bilder/Branchenl%C3%B6sungen/Verpackungstechnik/Etiketten800x500_-_Kopfbild-gr%C3%BCn.jpg)