Medical devices are subject to extremely strict quality requirements, to protect patients’ health to the greatest possible extent. This also applies to vials that are filled with powdered medicinal products, solutions or suspensions for use in medicine and chemical laboratories. These vials or ampoules are sealed with a rubber stopper or a PTFE septum (pierceable membrane).
To ensure that only absolutely defect-free vials are used, they must pass very strict quality controls both before and after filling. A cost-effective 100% inspection to ensure the vials are in perfect condition – including correct sealing and print or labelling – requires highly efficient automated inspection systems.
With vial.inspect, customers around the world put their trust in OCTUM’s modular 360° solution for inspecting vials throughout the filling process. Safe, fast and objective. Any questions? Call us or use our contact form – we’ll be pleased to help!

Any questions?
Get directly in touch with our
experts on +49 7062 91494-0
or write to us using our contact form
With vial.inspect, certified solution partner OCTUM offers reliable, flexible and high-performance systems for vial quality control, which have already proven their worth among numerous machine manufacturers and vaccine producers. vial.inspect therefore guarantees maximum safety of medicines.
- 100% in-process checks
- Inspections:
- Empty vial
- Vial mouth
- Stopper seat
- Crimp
- 21 CFR Part 11 and EU GMP compliant
- Includes user management and audit trails
- Modular for easy expansion
- Machine cycle time: up to 600 vials per minute
- New sizes can be set up quickly and flexibly
- Stainless steel enclosure
vial.inspect examines empty vials before they are filled with medicines or vaccines and reliably identifies any defects. The system is flexible and can be used for a huge range of vial sizes.
- Inspection zone: Base of vial
- Inspection characteristics:
- Contamination
- Foreign bodies
- Glass fragments
- Cracks
- Size range*: 2R – 50R
- Defect sizes**: approx. 0.2 x 0.2 mm
* Special sizes possible on request
** See defect catalogue
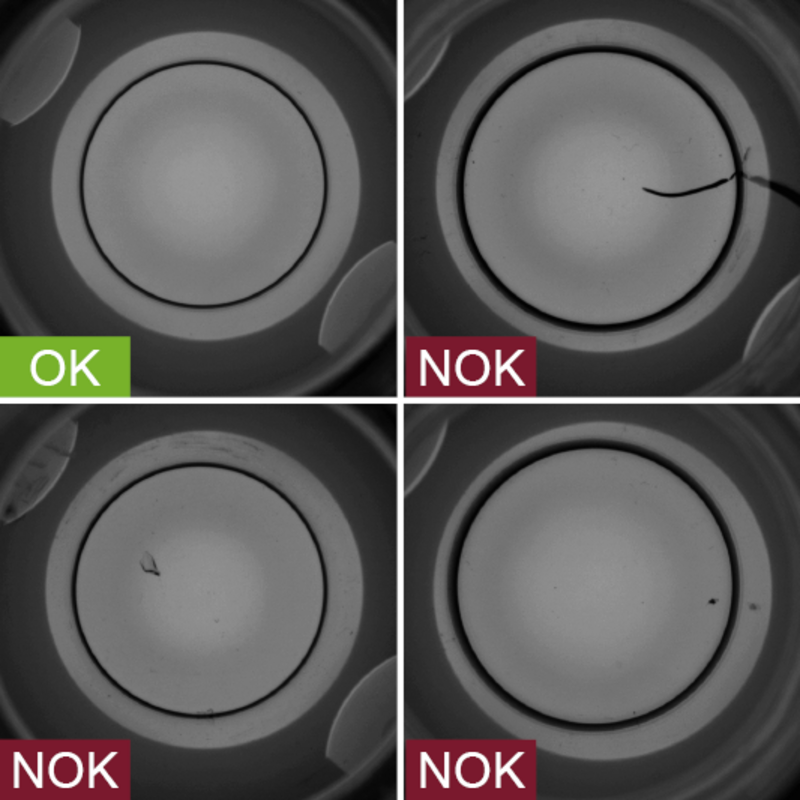
The mouths of vials are another source of possible defects, which vial.inspect can examine with precision before vials are filled with medicines and vaccines. Only absolutely defect-free vials are released for the downstream filling process.
- Inspection zone: Vial mouth
- Inspection characteristics:
- Damage
- Chips or flakes
- Impurities
- Size range*: 13 mm, 20 mm, 32 mm
- Defect sizes**: approx. 1.2 x 1.2 mm
- Special solution for mouths with oblique surfaces
* Special sizes possible on request
** See defect catalogue
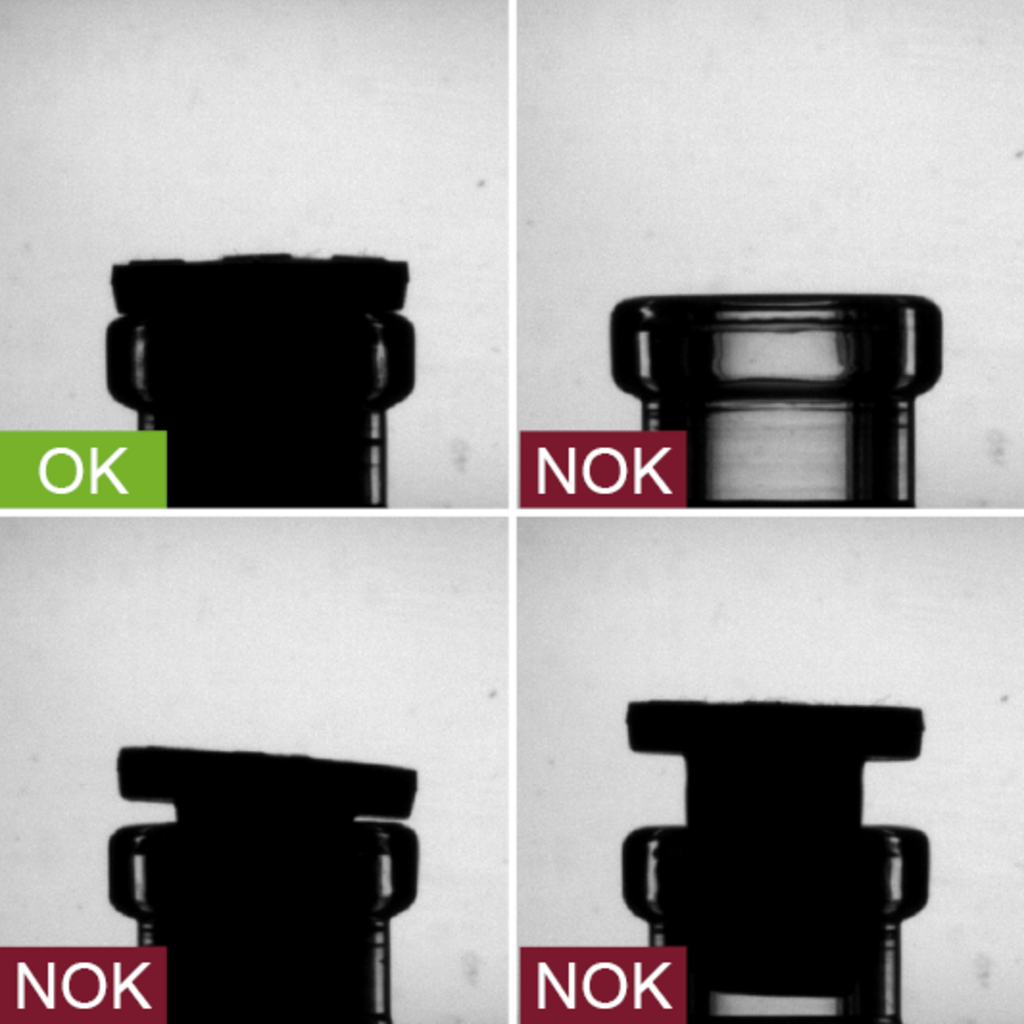
Incorrectly sealed vials can lead to contamination of the active ingredients and are a safety hazard. To prevent microbiological contamination, vial.inspect visually inspects the gap between the vial neck and stopper from two perspectives and reliably detects inadequately sealed vials.
- Inspection zone: Vial neck and stopper
- Inspection characteristics:
- Correctly seated stopper
- Gap between stopper and vial
- Stopper sizes: Common liquid and lyophilisation stoppers for ISO vials with an opening of 13 – 32 mm
- In a stainless steel housing with integrated light
- Space-saving location right above the stopper
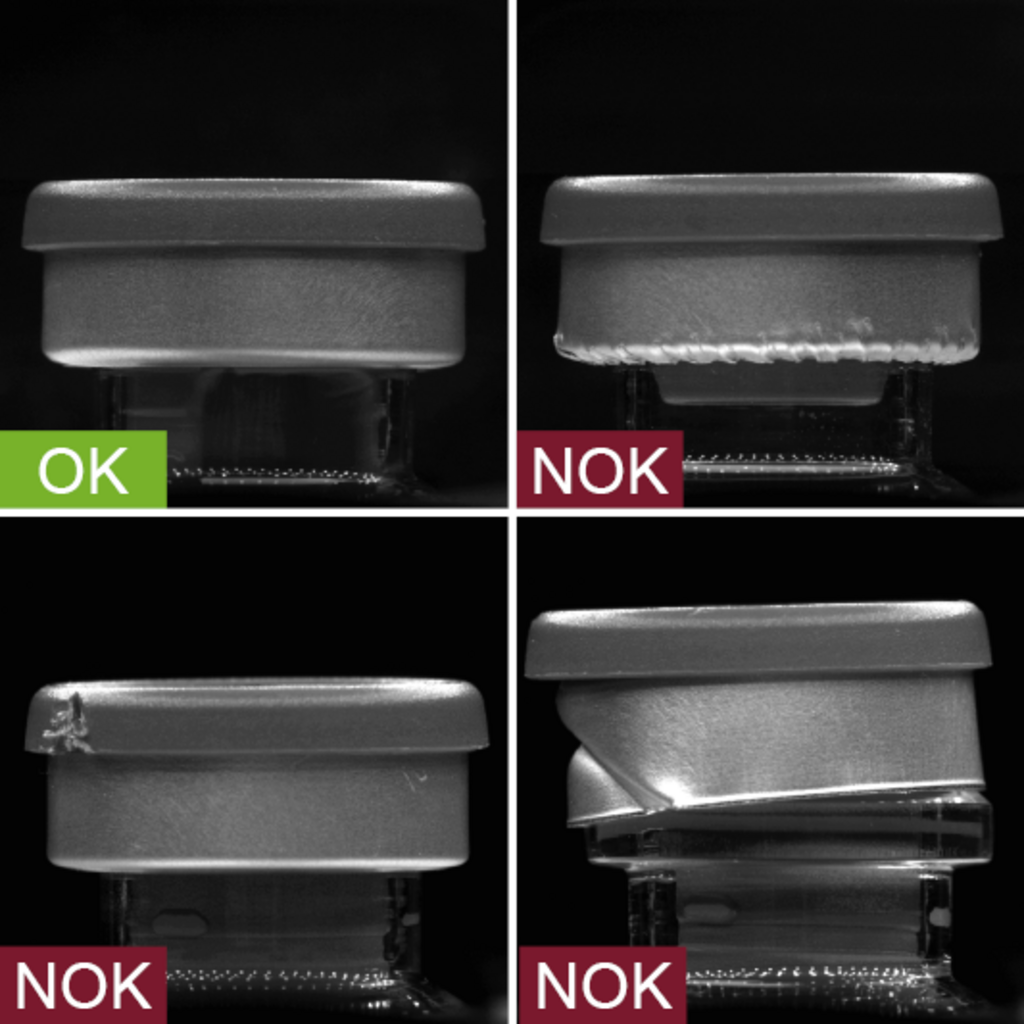
Crimp caps are metal caps that seal the vial stoppers after they have been filled, ensuring that the stoppers can no longer become detached. vial.inspect inspects these crimp caps from up to 4 perspectives simultaneously during a 100% inspection and makes sure that only perfectly sealed vials can continue on to downstream processes and then be delivered to customers.
- Inspection zone: Crimp cap
- Inspection characteristics:
- Crimp presence
- Crimp quality
- Damage to the crimp cap
- Presence of flip-off caps
- Damage to flip-off caps
- Colour of flip-off caps
- Size range: Common crimp caps for ISO vials with an opening of 13 – 32 mm
- In a stainless steel housing with integrated light
- Space-saving location right above the crimp cap
For clear identification, vials have variable data such as a batch or serial number printed on them, enabling reliable identification of individual batches and seamless traceability in the event of a recall. vial.inspect also covers this task, and checks the print to make sure that identification is present, legible and contains the correct information.
- Inspection zone: Crimp cap, flip-off cap, side of vial, label
- Inspection characteristics:
- Checking plain text such as the batch or serial number
- Verifying 1D or 2D codes such as data matrix codes and barcodes
- Variable data:
- Batch number
- Serial number
- Date of manufacture
- Expiry date
- Types of code: All common 1D and 2D codes (barcodes, data matrix, etc.)
- Printing methods: Laser, inkjet
- In a stainless steel housing with integrated light
- UV ink can also be checked as an option

![[Translate to Englisch:] pharmazeutische Vials bzw. Injektionsfläschchen werden in einer Abfüllanlage im Stern geführt](/fileadmin/user_upload/content-bilder/Branchenl%C3%B6sungen/Pharma/vial.inspect/Vials_800x500.jpg)